missing translation for 'onlineSavingsMsg'
Learn More
Learn More
Invitrogen™ Ebdarokimab Recombinant Monoclonal Antibody


Recombinant Monoclonal Antibody
Marke: Invitrogen™ MA559263
Dieser Artikel kann nicht zurückgegeben werden.
Rückgaberichtlinie anzeigen
Beschreibung
For reconstitution, add sterile, distilled water to achieve a final antibody concentration of 1 mg/mL. Gently shake to solubilize the protein completely. Do not vortex. Reconstituted products should be stored at -80 °.
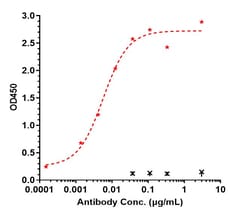
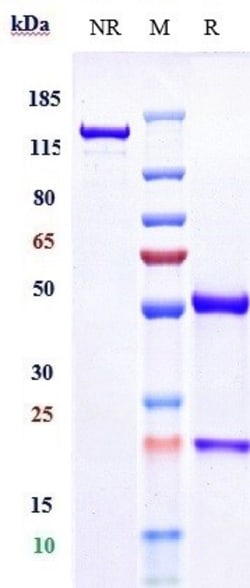
Ebdarokimab, also known as AK101, is a monoclonal antibody that targets interleukin-12 (IL-12) and interleukin-23 (IL-23), two cytokines implicated in the inflammatory response associated with plaque psoriasis. Interleukin-12 subunit beta (IL-12b), also known as IL-12p40, is a crucial component of the heterodimeric cytokine IL-12 and IL-23. The IL-12b gene is located on chromosome 5q31-33 and encodes for a 40 kDa protein. IL-12 is composed of two subunits, p35 and p40, while IL-23 is composed of p19 and the shared p40 subunit. The IL-12b protein has a four-helix bundle structure, homologous to other members of the hematopoietin superfamily. Functionally, IL-12b plays a significant role in immune responses by promoting the differentiation of naive T cells into Th1 cells and enhancing the cytotoxic activity of natural killer (NK) cells. It is essential in the immune system's response to intracellular pathogens. Dysregulation of IL-12b has been implicated in various autoimmune diseases and cancers, making it a target for therapeutic interventions.
Spezifikation
| Ebdarokimab Humanized | |
| Recombinant Monoclonal | |
| Unconjugated | |
| Human | |
| 100 μg | |
| Primary | |
| -20°C, Avoid Freeze/Thaw Cycles | |
| Lyophilized |
| ELISA, Flow Cytometry, Functional Assay, Surface Plasmon Resonance | |
| 1 mg/mL | |
| 25mM histidine with 8% sucrose, 0.01% Tween 80 and no preservative; pH 6.2 | |
| Protein A | |
| RUO | |
| Human | |
| Antibody | |
| IgG1 |
Berichtigung von Produktinhalten
Bitte geben Sie uns Ihr Feedback zu den Produktinhalten, indem Sie das folgende Formular ausfüllen.
Name des Produkts
Haben Sie Verbesserungsvorschläge?Übermitteln Sie eine inhaltliche Korrektur