missing translation for 'onlineSavingsMsg'
Learn More
Learn More
Invitrogen™ Ravulizumab Recombinant Monoclonal Antibody
Recombinant Monoclonal Antibody
Marke: Invitrogen™ MA559286
Dieser Artikel kann nicht zurückgegeben werden.
Rückgaberichtlinie anzeigen
Beschreibung
For reconstitution, add sterile, distilled water to achieve a final antibody concentration of 1 mg/mL. Gently shake to solubilize the protein completely. Do not vortex. Reconstituted products should be stored at -80 °.
Ravulizumab is considered a long-acting complement 5 (C5) inhibitor that has been undergoing clinical trials for the treatment of paroxysmal nocturnal hemoglobinuria (PNH) as of 4 February, 2016. A pharmaceutical similar to ravulizumab (ALXN1210), called eculizumab, is currently approved for the treatment of PNH in 46 countries under the brand name Soliris®. Ravulizumab is considered by Alexion Pharmaceuticals Inc. to be a next-generation eculizumab molecule. Ravulizumab was subsequently approved by the US FDA in December of 2018 for a variety of beneficial characteristics that make it an advanced, next-generation agent in comparison to eculizumab. In particular, ravulizumab is currently the first and only long-acting C5 complement inhibitor that can be administered every eight weeks for the treatment of adult patients with PNH whereas eculizumab is a bi-weekly treatment, Label. Moreover, virtually all of the phase 3 trial results for ravulizumab have demonstrated the equivalent efficacy and safety established by eculizumab and that patients transition safely and effectively from using eculizumab to ravulizumab. Subsequently, whereas PNH patients may have needed to previously plan their lives rather strictly around the bi-weekly infusion administrations of eculizumab, with ravulizumab such patients can find a more relaxed dosing schedule of only six or seven infusions over an entire year, Label.
Spezifikation
| Ravulizumab Humanized | |
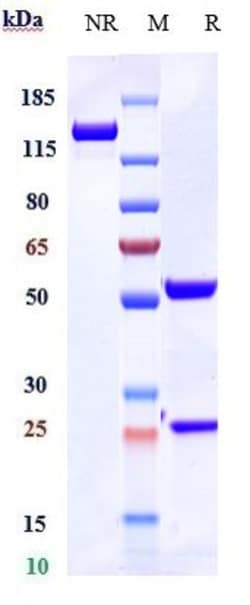
| Recombinant Monoclonal | |
| Unconjugated | |
| ALXN-1210; ravulizumab-cwvz | |
| Protein A | |
| RUO | |
| Human | |
| Antibody | |
| IgG2SA |
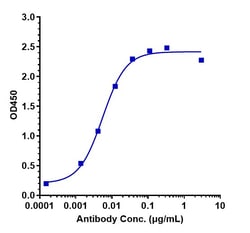
| ELISA, Flow Cytometry, Functional Assay, Surface Plasmon Resonance | |
| 1 mg/mL | |
| 25mM histidine with 8% sucrose, 0.01% Tween 80 and no preservative; pH 6.2 | |
| Human | |
| 1 mg | |
| Primary | |
| -20°C, Avoid Freeze/Thaw Cycles | |
| Lyophilized |
Berichtigung von Produktinhalten
Bitte geben Sie uns Ihr Feedback zu den Produktinhalten, indem Sie das folgende Formular ausfüllen.
Name des Produkts
Haben Sie Verbesserungsvorschläge?Übermitteln Sie eine inhaltliche Korrektur