missing translation for 'onlineSavingsMsg'
Learn More
Learn More
Invitrogen™ Teprotumumab Recombinant Monoclonal Antibody


Beschreibung
For reconstitution, add sterile, distilled water to achieve a final antibody concentration of 1 mg/mL. Gently shake to solubilize the protein completely. Do not vortex. Reconstituted products should be stored at -80 °.
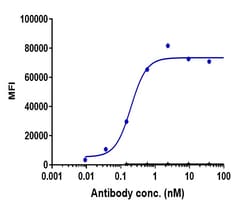
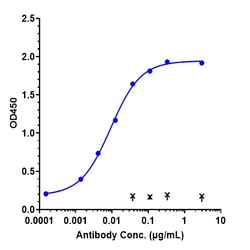
Teprotumumab is a fully human IgG1 monoclonal antibody directed against the human insulin-like growth factor-1 receptor. 8 Following a clinical trial in which its efficacy in the treatment of thyroid eye disease (TED) was assessed, it received breakthrough therapy designation from the FDA in 20163 and was approved by the FDA in January 2020 for the treatment of TED. 7 Thyroid eye disease is a potentially debilitating complication of Graves' Disease involving inflammation and tissue remodeling behind the eye, and previous treatment options typically involved multiple invasive surgeries - teprotumumab is the first pharmaceutical ever approved for the treatment of TED and therefore represents a significant step forward in the treatment this disease.

Spezifikation
Spezifikation
| Antigen | Teprotumumab |
| Anwendungen | ELISA, Flow Cytometry, Functional Assay, Surface Plasmon Resonance |
| Klassifikation | Recombinant Monoclonal |
| Konzentration | 1 mg/mL |
| Konjugat | Unconjugated |
| Zusammensetzung | 25mM histidine with 8% sucrose, 0.01% Tween 80 and no preservative; pH 6.2 |
| Gen-Alias | RO4858696-000 |
| Wirtsspezies | Human |
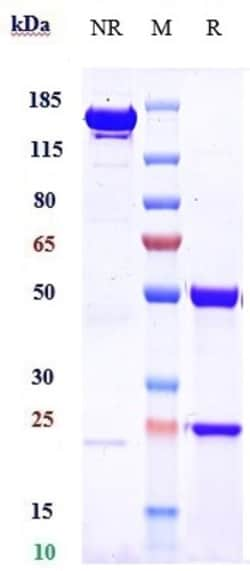
| Reinigungsverfahren | Protein A |
| Menge | 100 μg |
| Mehr anzeigen |
Name des Produkts
Indem Sie auf Absenden klicken, erklären Sie sich damit einverstanden, dass Fisher Scientific sich mit Ihnen in Verbindung setzen kann, um Ihr Feedback in diesem Formular zu bearbeiten. Wir werden Ihre Informationen nicht für andere Zwecke weitergeben. Alle bereitgestellten Kontaktinformationen werden in Übereinstimmung mit unserer Datenschutzrichtlinie aufbewahrt. Datenschutzrichtlinie.
Haben Sie Verbesserungsvorschläge?