missing translation for 'onlineSavingsMsg'
Learn More
Learn More
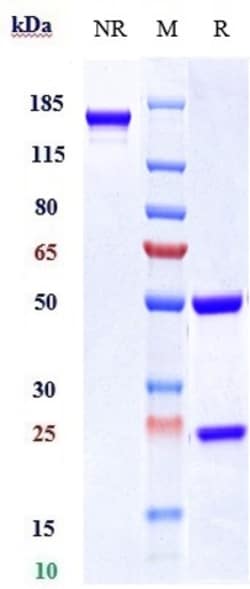
Invitrogen™ Tildrakizumab Recombinant Monoclonal Antibody


Recombinant Monoclonal Antibody
477.00 € - 1204.00 €
Spezifikation
| Antigen | Tildrakizumab Humanized |
|---|---|
| Konzentration | 1 mg/mL |
| Inhalt und Lagerung | -20°C, Avoid Freeze/Thaw Cycles |
| Anwendungen | ELISA, Flow Cytometry, Functional Assay, Surface Plasmon Resonance |
| Klassifikation | Recombinant Monoclonal |
| Product Code | Brand | Menge | Price | Quantity & Availability | |||||
|---|---|---|---|---|---|---|---|---|---|
| Product Code | Brand | Menge | Price | Quantity & Availability | |||||
30283058
 |
Invitrogen™
MA558659 |
100 μg |
477.00 €
100 Mikrogramm |
Log in om dit product te kopen Registreer vandaag om een webaccount aan te maken | |||||
|
30282438
|
Invitrogen™
MA558660 |
1 mg |
1204.00 €
1 Milligramm |
Log in om dit product te kopen Registreer vandaag om een webaccount aan te maken | |||||
Description
For reconstitution, add sterile, distilled water to achieve a final antibody concentration of 1 mg/mL. Gently shake to solubilize the protein completely. Do not vortex. Reconstituted products should be stored at -80 °.
Tildrakizumab is a high-affinity, humanized, IgG1 kappa antibody targeting interleukin 23 p19 that shows promise in the evolution of treatment strategy in chronic plaque psoriasis 3. The Food and pharmaceutical Administration (FDA) approved ILUMYA (tildrakizumab-asmn) for the treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy in March 2018. The approved recommended dosage of ILUMYA is a subcutaneous injection of 100 mg at Weeks 0, 4, and every 12 weeks thereafter 7. A study was performed on the pharmacokinetics of this pharmaceutical on various ethnicities. The pharmacokinetics of tildrakizumab were similar in Japanese, Caucasian, and Chinese subjects 5.Specifications
| Tildrakizumab Humanized | |
| -20°C, Avoid Freeze/Thaw Cycles | |
| Recombinant Monoclonal | |
| Lyophilized | |
| IgG1 | |
| Human | |
| SCH900222; MK-3222 | |
| Antibody |
| 1 mg/mL | |
| ELISA, Flow Cytometry, Functional Assay, Surface Plasmon Resonance | |
| Unconjugated | |
| Human | |
| RUO | |
| 25mM histidine with 8% sucrose, 0.01% Tween 80 and no preservative; pH 6.2 | |
| Primary | |
| Protein A |
Spot an opportunity for improvement?Share a Content Correction
Product Content Correction
Your input is important to us. Please complete this form to provide feedback related to the content on this product.
Product Title